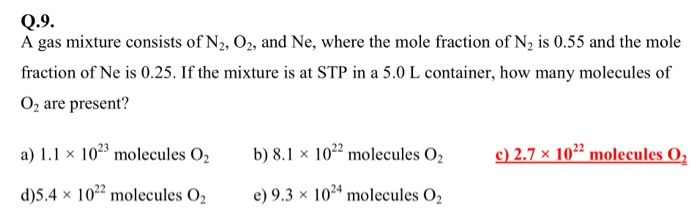
35+ pages a gas mixture consists of n2 o2 and ne 725kb. Determine the heat transfer during this process per mole of the mixture using a the ideal-gas. A gas mixture consists of n2 o2 and ne A gas mixture consists of N2 O2 and Ne where the mole fraction of N2 isA gas mixture consists of N2 O2 and Ne where the mole fraction of N2 is 055 and the mole fraction of Ne is 025. If the mixture is at STP in a 50 L container how many molecules of O2 are present. Read also consists and understand more manual guide in a gas mixture consists of n2 o2 and ne If the mixture is at STP in a 50 L container how many molecules of O2 are present.
Answer to A gas mixture consists of O2 and N2. A gas mixture consists of O2 and N2.

A Mixture Of Gases At 760 Mm Pressure Contains 65 Nitrogen 15 Oxygen And 20 Carbon Dioxide Volume What Is Partial Pressure Of Each In Mm
| Title: A Mixture Of Gases At 760 Mm Pressure Contains 65 Nitrogen 15 Oxygen And 20 Carbon Dioxide Volume What Is Partial Pressure Of Each In Mm |
| Format: PDF |
| Number of Pages: 169 pages A Gas Mixture Consists Of N2 O2 And Ne |
| Publication Date: June 2017 |
| File Size: 1.4mb |
| Read A Mixture Of Gases At 760 Mm Pressure Contains 65 Nitrogen 15 Oxygen And 20 Carbon Dioxide Volume What Is Partial Pressure Of Each In Mm |
 |
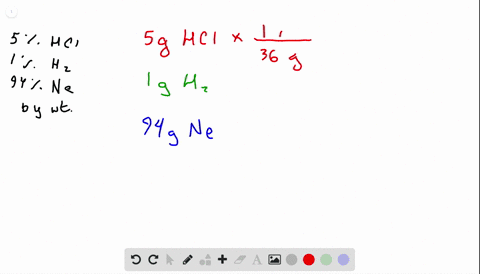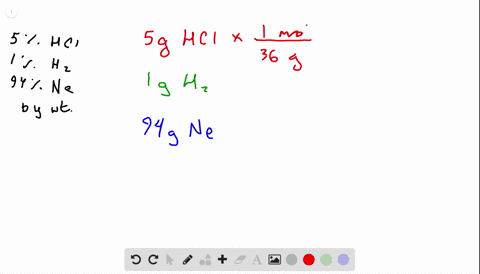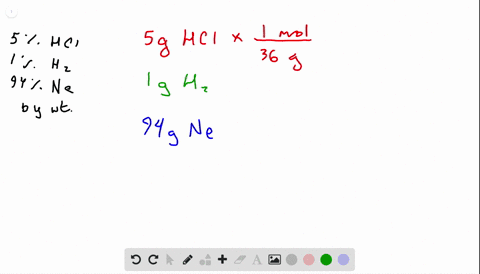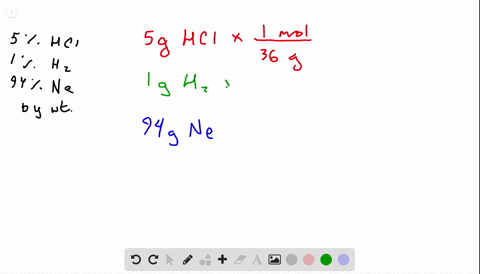
A gas mixture consists of N2 O2 and Ne where the mole fraction of N2 is 055 and the mole fraction of Ne is 025.
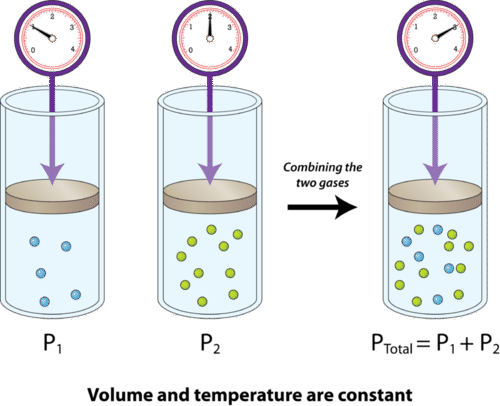
Neglecting all vibrational modes the total internal energy of the system is Neglecting all vibrational modes the total internal energy of. If the mixture is at STP in a 50 L container how many molecules of 02 are present. 269x10 molecules of O. If the mixture is at STP in a 50 L contaier how many molecules of 02 are present. A gas mixture consists of N2 O2 and Ne where the mole fraction of N2 is 055 and the mole fraction of Ne is 025. A 45 1022 molecules O2 B 27 1022 molecules O2 C 37 1023 molecules O2 D 11 1023 molecules O2 E 93 1024 molecules O2.
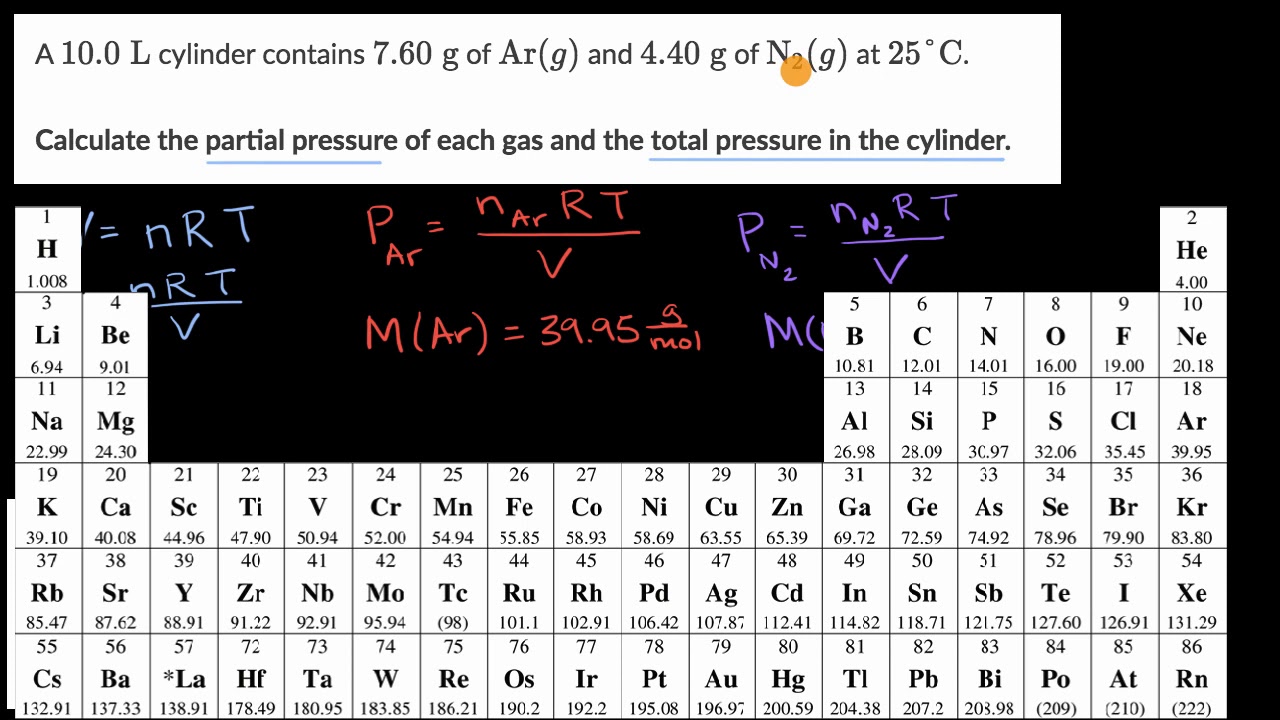
Calculating Partial Pressures Worked Example Video Khan Academy
| Title: Calculating Partial Pressures Worked Example Video Khan Academy |
| Format: ePub Book |
| Number of Pages: 262 pages A Gas Mixture Consists Of N2 O2 And Ne |
| Publication Date: July 2017 |
| File Size: 1.8mb |
| Read Calculating Partial Pressures Worked Example Video Khan Academy |
 |
Solved What Is The Mole Fraction Of Each Ponent In A Mixture Of 12 45 Mathrm G Of Mathrm H 2 60 67 Mathrm G
| Title: Solved What Is The Mole Fraction Of Each Ponent In A Mixture Of 12 45 Mathrm G Of Mathrm H 2 60 67 Mathrm G |
| Format: PDF |
| Number of Pages: 239 pages A Gas Mixture Consists Of N2 O2 And Ne |
| Publication Date: December 2019 |
| File Size: 2.8mb |
| Read Solved What Is The Mole Fraction Of Each Ponent In A Mixture Of 12 45 Mathrm G Of Mathrm H 2 60 67 Mathrm G |
 |
Q 9 A Gas Mixture Consists Of N2 O2 And Ne Where Chegg
| Title: Q 9 A Gas Mixture Consists Of N2 O2 And Ne Where Chegg |
| Format: eBook |
| Number of Pages: 330 pages A Gas Mixture Consists Of N2 O2 And Ne |
| Publication Date: February 2017 |
| File Size: 2.8mb |
| Read Q 9 A Gas Mixture Consists Of N2 O2 And Ne Where Chegg |
 |
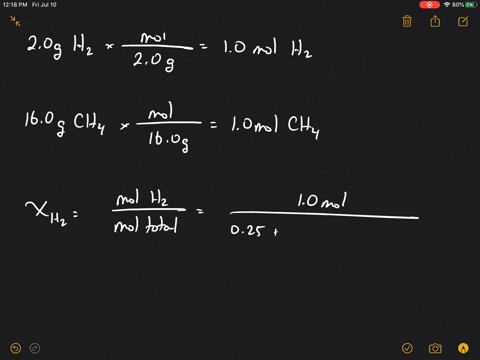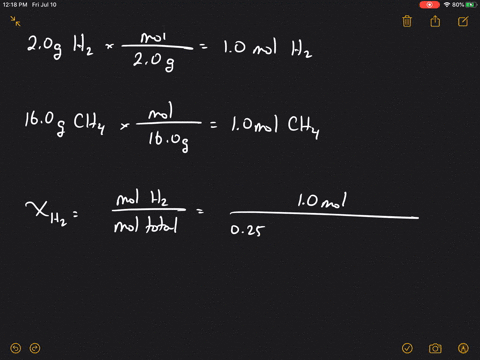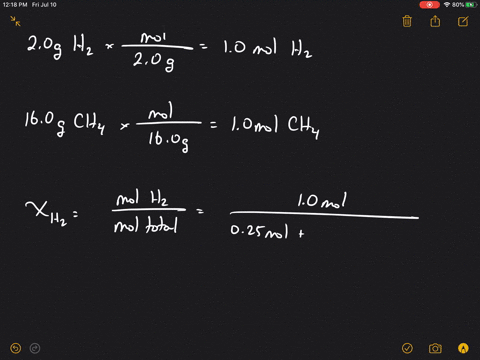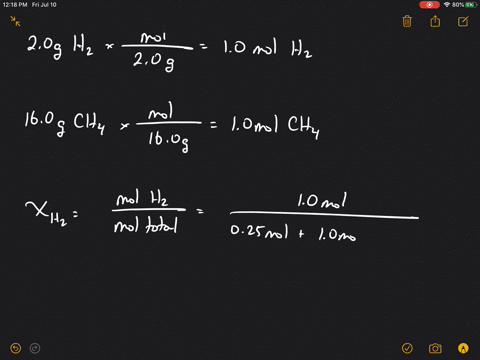
Solved What Is The Mole Fraction Of Each Ponent In A Mixture Of 12 45 Mathrm G Of Mathrm H 2 60 67 Mathrm G
| Title: Solved What Is The Mole Fraction Of Each Ponent In A Mixture Of 12 45 Mathrm G Of Mathrm H 2 60 67 Mathrm G |
| Format: ePub Book |
| Number of Pages: 142 pages A Gas Mixture Consists Of N2 O2 And Ne |
| Publication Date: February 2019 |
| File Size: 800kb |
| Read Solved What Is The Mole Fraction Of Each Ponent In A Mixture Of 12 45 Mathrm G Of Mathrm H 2 60 67 Mathrm G |
 |
S Onlinelibrary Wiley Doi Pdf 10 1002 9783527621248 Ch2
| Title: S Onlinelibrary Wiley Doi Pdf 10 1002 9783527621248 Ch2 |
| Format: eBook |
| Number of Pages: 241 pages A Gas Mixture Consists Of N2 O2 And Ne |
| Publication Date: September 2020 |
| File Size: 2.2mb |
| Read S Onlinelibrary Wiley Doi Pdf 10 1002 9783527621248 Ch2 |
 |

21 A Vessel Contained N2 Ar He And Ne The Total Chegg
| Title: 21 A Vessel Contained N2 Ar He And Ne The Total Chegg |
| Format: eBook |
| Number of Pages: 199 pages A Gas Mixture Consists Of N2 O2 And Ne |
| Publication Date: April 2020 |
| File Size: 1.8mb |
| Read 21 A Vessel Contained N2 Ar He And Ne The Total Chegg |
 |
S Mdpi 2076 3417 9 24 5429 Pdf
| Title: S Mdpi 2076 3417 9 24 5429 Pdf |
| Format: ePub Book |
| Number of Pages: 234 pages A Gas Mixture Consists Of N2 O2 And Ne |
| Publication Date: January 2020 |
| File Size: 1.3mb |
| Read S Mdpi 2076 3417 9 24 5429 Pdf |
 |
Gas Mixtures Ck 12 Foundation
| Title: Gas Mixtures Ck 12 Foundation |
| Format: PDF |
| Number of Pages: 302 pages A Gas Mixture Consists Of N2 O2 And Ne |
| Publication Date: June 2021 |
| File Size: 2.6mb |
| Read Gas Mixtures Ck 12 Foundation |
 |
The Reaction N2 G O2 G 2no G Contributes To Air Pollution Whenever A Fuel Is Burnt In Air At A High Temperature At 1500k Equilibrium Constant K For It Is 1 0 10 5
| Title: The Reaction N2 G O2 G 2no G Contributes To Air Pollution Whenever A Fuel Is Burnt In Air At A High Temperature At 1500k Equilibrium Constant K For It Is 1 0 10 5 |
| Format: ePub Book |
| Number of Pages: 266 pages A Gas Mixture Consists Of N2 O2 And Ne |
| Publication Date: April 2019 |
| File Size: 1.9mb |
| Read The Reaction N2 G O2 G 2no G Contributes To Air Pollution Whenever A Fuel Is Burnt In Air At A High Temperature At 1500k Equilibrium Constant K For It Is 1 0 10 5 |
 |

Solved What Is The Mole Fraction Of Each Ponent In A Mixture Of 12 45 Mathrm G Of Mathrm H 2 60 67 Mathrm G
| Title: Solved What Is The Mole Fraction Of Each Ponent In A Mixture Of 12 45 Mathrm G Of Mathrm H 2 60 67 Mathrm G |
| Format: PDF |
| Number of Pages: 196 pages A Gas Mixture Consists Of N2 O2 And Ne |
| Publication Date: May 2021 |
| File Size: 2.1mb |
| Read Solved What Is The Mole Fraction Of Each Ponent In A Mixture Of 12 45 Mathrm G Of Mathrm H 2 60 67 Mathrm G |
 |
S Mdpi 2076 3417 9 24 5429 Pdf
| Title: S Mdpi 2076 3417 9 24 5429 Pdf |
| Format: eBook |
| Number of Pages: 213 pages A Gas Mixture Consists Of N2 O2 And Ne |
| Publication Date: December 2020 |
| File Size: 800kb |
| Read S Mdpi 2076 3417 9 24 5429 Pdf |
 |
If the mixture is at STP in a 50 L container how many molecules of 02 are present. The sum of mole fractions in a. The ratio of the mole numbers of N2 to O2 is 31.
Here is all you have to to read about a gas mixture consists of n2 o2 and ne E is at STP in a 50 L container how many molecules of O2 are present. This mixture is heated during a steady-flow process from 180 to 210 K at a constant pressure of 8 MPa. Log in Sign up. S onlinelibrary wiley doi pdf 10 1002 9783527621248 ch2 calculating partial pressures worked example video khan academy the reaction n2 g o2 g 2no g contributes to air pollution whenever a fuel is burnt in air at a high temperature at 1500k equilibrium constant k for it is 1 0 10 5 gas mixtures ck 12 foundation s mdpi 2076 3417 9 24 5429 pdf s mdpi 2076 3417 9 24 5429 pdf See the answer See the answer done loading.



0 Comments